thrombomiR™ microRNA qPCR test kit
TAmiRNA’s new thrombomiRTM microRNA qPCR kit provides a novel but easy to use tool for analyzing platelet-function using circulating plasma miRNAs. The kit enables standardized analysis of platelet-derived microRNA to determine platelet function in vivo. Based on a simple technology platform the kit can easily be adopted by genetic test laboratories and testing can be performed on as little as only 200 μL of fresh or frozen plasma. It offers interesting opportunities for clinical research groups and pharmaceutical companies that are active in platelet function research, anti-platelet therapy and age-related/cardiovascular diseases worldwide.
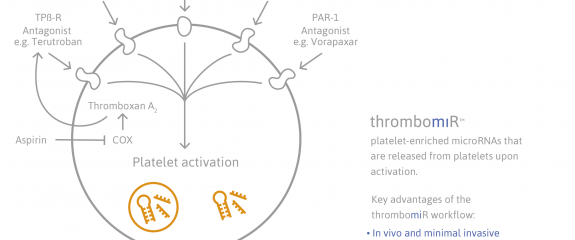
Platelets contain miRNAs and the expression profiles within platelets correlate with their activity. During activation, platelets release microparticles containing miRNAs that are then available for uptake by other cells, allowing direct regulation of the recipient cell´s mRNA profile and gene expression. Consequently, circulating miRNA, plasma and serum miRNA profiles can also be used as biomarkers of prognosis and treatment response. Dysregulation and inappropriate activation of platelets occurs in a wide range of serious age-associated diseases, including stroke and myocardial infraction. As they play a key role in hemostasis, initiating and propagating thrombosis better understanding of a patient's platelet 'status' can provide insight for clinical decision making relating to bleeding disorders and additional risk during surgery.
The understanding of biomarkers as indicators of platelet function has matured to become one of the first validated diagnostic applications of this technology. There are three potential benefits to using quantitative analysis of circulating miRNAs in human plasma as a measure of platelet function:
- miRNA circulation reflects a platelet function that is independent of any specific activation pathway
- indication of in-vivo function of platelets
- measurements can be performed on deep-frozen samples
- it provides an in vivo measure of platelet function that is independent of the activation pathway
The thrombomiRTM kit’s development originated from an academic research project conducted at the laboratory of Dr. Manuel Mayr at the King´s British Heart Foundation Centre, King´s College London, UK, which produced a blood-based biomarker signature as a novel measure of platelet function.
The thrombomiRTM workflow is intended for research-use as an outcome measure in observational or interventional clinical trials. The kit enables simple and rapid analysis of thrombomiRs, which are circulating microRNAs that reflect platelet function. ThrombomiRs enable personalized monitoring of anti-platelet therapy, and the assessment of bleeding-risk due to impaired platelet activation. Since platelet function is an important contributor to cardiovascular disease, utility for bleeding risk due to traumatic events, risk assessment of adverse cardiovascular events and early diagnosis of type-2 diabetes have been proposed.
Proposed benefits of the thrombomiRTM qPCR kit include:
- Rapid test results
- Minimal invasiveness
- Easy, standardized workflow for circulating microRNA analysis
- Sophisticated panel of controls to generate high quality data
- High sensitivity and specificity
- Comparability of data due to standardized data processing and normalization
- Accessible to laboratories and clinics lacking facilities for point-of-care testing
Product testing of its utility in cardiometabolic diseases is already underway.


